Dr. Dianne M. Walters, Ph.D.

Assistant Professor
Phone: 252-744-5453
E-mail: waltersd@ecu.edu
Research Interests
Idiopathic pulmonary fibrosis (IPF) is a complex interstitial lung disease characterized by chronic, progressive scarring of the lung thought to be triggered by ongoing epithelial injury. The etiology and pathogenic mechanisms underlying IPF are largely unknown, and despite intensive research, effective therapies have not been developed. Exposure to environmental agents in genetically susceptible individuals is thought to underlie development of IPF. Thus, to investigate gene x environment interactions in pulmonary fibrosis, we developed a mouse model of interstitial pulmonary fibrosis using vanadium pentoxide (V2O5), a transition metal found in ambient particulate matter and certain occupational settings. Haplotype mapping and genetic linkage analysis of V2O5-induced lung collagen content in mice identified several quantitative trait loci (QTL) and candidate susceptibility genes.
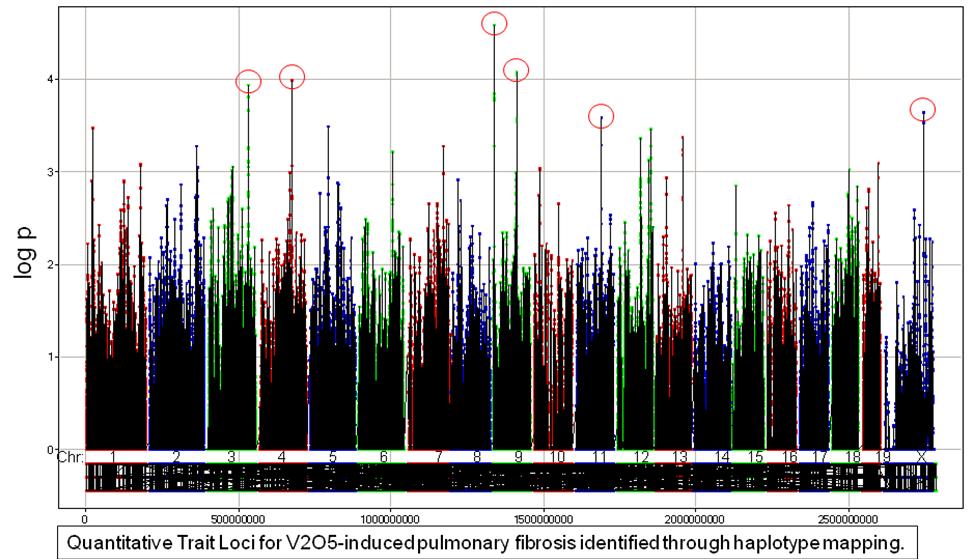
Our laboratory is currently further characterizing the V2O5-induced interstitial pulmonary fibrosis model and investigating the role of novel candidate susceptibility genes identified in QTLs.
- Characterization of the V2O5-induced interstitial pulmonary fibrosis model in mice. The lack of mechanistic understanding and effective therapies for IPF is partially attributed to the fact that animal models of pulmonary fibrosis do not closely mimic the human disease. We are investigating whether the V2O5 model is an improvement over other commonly used models in regard to progression and stability of fibrosis, histologic pattern of IPF, and biomarkers of fibrosis.
- Candidate susceptibility genes for fibrosis.
- A highly suggestive QTL on mouse chromosome 4 contains a gene which regulates particular microRNAs (miRNAs) that have been shown to be down regulated in IPF patients. Sequence analysis revealed a non-synonymous coding SNP in this gene in susceptible, but not resistant strains of mice. We hypothesize that this gene variant selectively reduces maturation of key miRNAs leading to enhanced expression of pro-fibrotic mediators and initiates a positive feedback loop, leading to phenotypic features of pulmonary fibrosis and disease progression.
- Another QTL contains a cluster of genes about which little is known; however, these genes appear to play a role in cell cycle regulation. We hypothesize that SNPs in these genes or their promotors in susceptible strains of mice interfere with the normal anti-proliferative function of these genes leading to cell cycle progression, fibroblast proliferation, and excessive deposition of extracellular matrix that characterizes fibrosis.
- Other candidate susceptibility genes await investigation.
Employment/Education
- 1991-1996
Research Biologist, U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), Frederick, MD - 1996-2002
Ph.D. The Johns Hopkins Bloomberg School of Public Health Dept. of Environmental Health Sciences, Physiology Division - 2002-2003
Intramural Research Training Award, Postdoctoral Fellow, NIEHS Laboratory of Respiratory Biology, Airway Inflammation Group - 2004-2008
Intramural Research Training Award, Postdoctoral Fellow, NIEHS Laboratory of Respiratory Biology, Environmental Genetics Group - 2008-present
Assistant Professor, Department of Physiology, Brody School of Medicine, East Carolina University
Professional Societies
- American Thoracic Society
2008 – present - American Physiological Society
2010 – present - North Carolina Society of Toxicology
2010 – present
Selected Publications
- Walters DM, Breysse PN, and Wills-Karp M. 2001. Ambient urban Baltimore particulate-induced airway hyperresponsiveness and inflammation in mice. Am. J. Respir. Crit. Care Med. 164(8): 1438-1443.
- Walters DM, Breysse PN, Schofield B, and Wills-Karp M. 2002. Complement factor 3 mediates particulate matter-induced airway hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 27: 413-418.
- Wang YZ, Ingram JL, Walters DM, Rice AB, Santos JH, Van Houten B, and Bonner JC. 2003. Vanadium-induced STAT-1 activation in lung myofibroblasts requires H2O2 and P38 MAP kinase. Free Radic Biol Med 35(8):845-55.
- Walters DM and Bonner JC. 2005. Signal transduction and cytokine expression in particulate matter (PM)-induced airway remodeling. In Air Pollutants and the Respiratory Tract. 2nd ed. Edited by WM Foster and DL Costa. Vol 204 of series Lung Biology in Health and Disease. Exec editor Claude Lenfant, publisher: Taylor and Francis Group.
- Walters DM, Antao-Menezes A, Ingram JL, Rice AB, Nyska A, Tani Y, Kleeberger SR, and Bonner JC. 2005. Susceptibility of signal transducer and activator of transcription-1-deficient mice to pulmonary fibrogenesis. Am J Pathol 167(5):1221-9.
- Walters DM, Cho HY, nd Kleeberger SR. 2008. Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: a potential role for NRF2. Antioxid. Redox Signal. 10(2):321-32.
- Walters DM, and Kleeberger SR. 2008. Bleomycin-induced pulmonary fibrosis. Current Protocols in Pharmacol. Supp#10;Unit 5.46.
- Rondini EA, Walters DM, Bauer AK. 2010. Vanadium pentoxide induces pulmonary inflammation and tumor promotion in a strain-dependent manner. Particle and Fibre Toxicology 7:9.
- Wingard CJ, Walters DM, Cathey B, Hilerbrand SC, Katwa P, Lin S, Ke PC, Podila R, Rao A, Lust RM, and Brown JM. 2010. Mast cells contribute to altered vascular reactivity and ischemia-reperfusion injury following cerium oxide nanoparticle instillation. Nanotox. Nov 3. 1-15 (e-pub ahead of print).